-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifugation Consumables
- Accessories
- Tubes
- Plates
- Device Management Software
- Sample and Information Management
- IVD Products

Control and Analytics
Lab Academy
- Bioprocessing
- Microbiology
- Cell Biology
- Cell Culture
- Culture of Microorganisms
- Bioprocess
- Essay
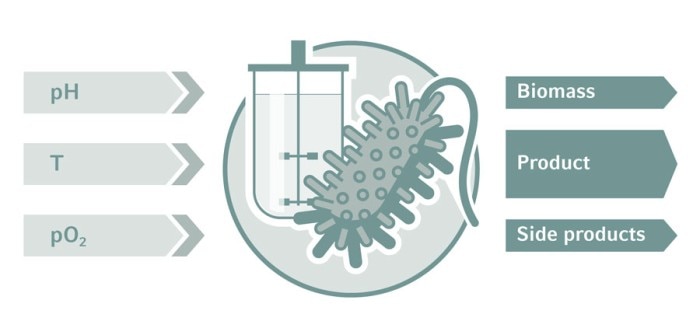
Monitoring and control is key in bioprocess development. Critical process parameters define the process environment for optimum cell growth and high-titer production. Control loops for temperature, dissolved oxygen (DO), pH, agitation, and level are routinely applied in bioreactor systems. Process Analytical Technology (PAT) provides deeper insights into the metabolic state of the culture and facilitates automation. Through the seamless integration of autosamplers and analyzers such as biomass monitors, cell counters, mass spectrometers, and HPLC, process engineers advance their development and work in line with Quality-by-Design (QbD) guidelines. Design of experiments (DoE) and multivariate analysis (MVA) are statistical tools to streamline experiments, which we discuss here in more detail.
Start it right: Design of experiments in bioprocess development
Biopharmaceutical production processes are influenced by a considerable number of parameters (also known as factors). In bioprocess development, these parameters are varied to increase efficiency and/or product quality. Popular experimental concepts range from trial and error and one-factor-at-a-time methods to structured approaches following Quality-by-Design (QbD) standards. QbD aims to design product quality and process efficiency into the process as early as possible [1]. When several critical process parameters are evaluated at once, possible interdependencies can be detected. The Design of Experiments (DoE) approach saves money and valuable development time.Read more
Read less
Setting the design space
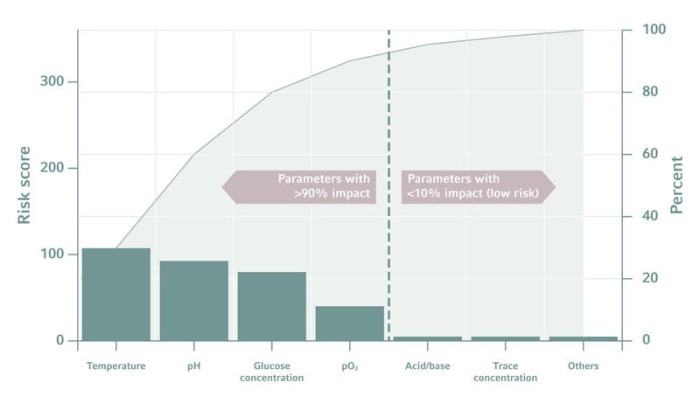
Picking the right parameters is the first, tricky step when designing the experimental setup. Researchers will find it easy to come up with a list of candidates, but how can we identify the most promising ones? The more we know about the process, the easier this will be. A Pareto chart can also be helpful to judge the importance of a parameter. Fortunately, only a few parameters have a major impact on the desired results!Read more
Read less
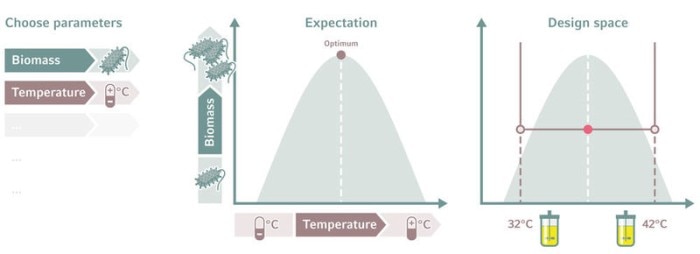
Output parameters also need to be considered when setting the design space, not just the input parameters. The response must be measurable, repeatable, and reproducible. You must decide at the outset how much output variability will be acceptable. In a typical bioprocess, a 5–10 % variability is tolerated [2].
Read more
Read less
Use your resources wisely
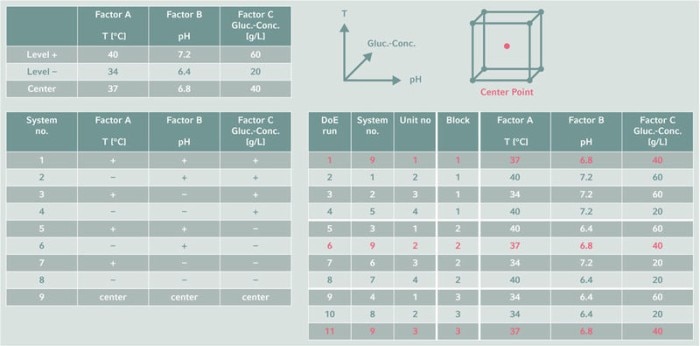
Once upper and lower setpoints are defined for each parameter, the experimental conditions must be mapped to the available resources. Parallel bioreactor systems allow multiple, simultaneous DoE runs, saving a lot of time compared to sequential runs. Where possible, resource mapping should be automated to reduce the risk of errors through manual operations, and to make documentation easier. And randomization of runs excludes hardware and environmental effects that might influence the results.Read more
Read less
Get the most out of your data—and don’t get lost
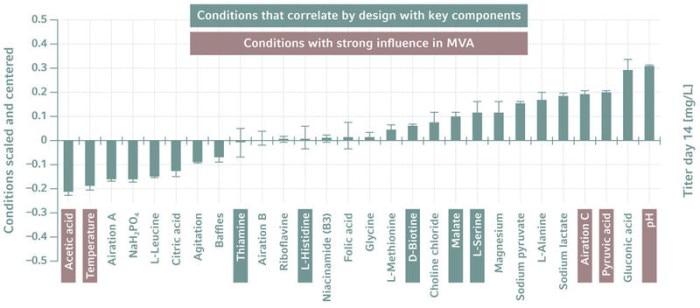
There are several software packages on the market for multivariate analysis (MVA) and DoE. Bioprocess data can be imported into these tools for evaluation. The packages use statistical methods to find the best combination of input factors, to describe their effect on the examined output factors, and to help us to understand how the parameters interact. They thus provide a starting point for further iterations.One popular modeling approach is principal component analysis (PCA), a method for identifying a smaller number of uncorrelated variables, called "principal components", from a large set of data. The goal is to explain the maximum amount of variance with the fewest principal components.
Another approach, used more often, is the partial least squares (PLS) method, which reduces the predictors to a smaller set of uncorrelated components and performs least squares regression on them, rather than the original data. Both approaches are introduced in [3].
Read more
Read less
References:
1. ICH (2009): Harmonised Tripartite Guideline. Pharmaceutical Development. Q8 (R2); https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf
2. Shivhare M, McCreath G (2010): Practical Considerations for DoE Implementation in Quality by Design. Bioprocess International Vol. 8, No. 6, June 2010, pp. 22 – 30
3. Glassey J (2016): Multivariate Modeling for Bioreactor Monitoring and Control. In: Bioreactors. Design, Operation and Novel Applications. Weinheim
Read more
Read less

